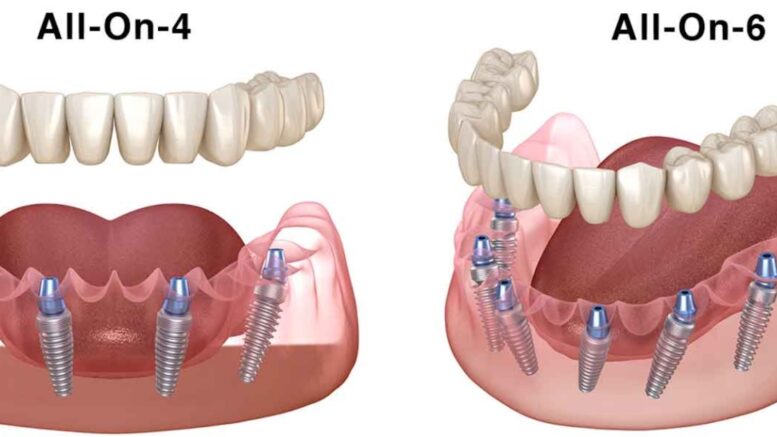
Dental laboratories today rarely work with a single implant system. As implant dentistry has matured, clinics have adopted different platforms based on training, availability, patient history, and surgical preference. For labs, this reality creates a daily challenge, restoring cases that involve multiple implant systems while maintaining accuracy, efficiency, and predictable outcomes.
Managing implant compatibility has become a core part of modern lab workflows. Rather than treating each system as a completely separate process, successful labs have developed strategies that allow them to restore diverse implant platforms without excessive inventory, delays, or technical risk.
The Reality of Multi-System Restorations
Most laboratories receive cases from multiple clinics, each with their own implant preferences. A single week may include restorations for internal hex systems, conical connections, and legacy platforms that are no longer actively marketed but still common in patient mouths.
This variety increases complexity. Each system may require different abutments, screws, analogs, and restorative protocols. Without a clear strategy, labs can experience bottlenecks, incorrect component selection, and extended turnaround times.
To remain competitive, labs must streamline how they handle this diversity while preserving restorative accuracy.
Compatibility as a Workflow Solution
Compatibility in implant components has become an important tool for laboratories managing multiple systems. High-quality compatible components are designed to replicate original implant geometries with precise machining tolerances. When done correctly, compatibility allows labs to restore a wide range of systems using consistent workflows.
This approach reduces the need to stock numerous system-specific parts and allows technicians to focus on restoration design rather than component sourcing. Compatibility also helps labs standardize internal protocols, which is especially valuable in high-volume environments.
Inventory Management and Efficiency
One of the biggest advantages of compatibility is inventory control. Stocking original components for every implant platform can tie up capital and shelf space, especially when some systems are used infrequently.
By relying on compatible components that cover widely used platforms, labs can reduce inventory complexity while still supporting the needs of their clinical partners. This leads to faster case processing, fewer emergency orders, and more predictable delivery timelines.
Efficient inventory management directly impacts lab profitability, especially as margins tighten across the dental industry.
Accuracy Still Comes First
Compatibility does not eliminate the need for precision. For a lab, restorative success depends on accurate seating, stable screw engagement, and consistent connection geometry. Poorly manufactured components can introduce micro movement, compromise margins, and increase the risk of remakes.
Reputable compatible components are produced using CNC machining, controlled quality processes, and biocompatible materials such as titanium alloys. These standards help ensure that compatibility supports clinical outcomes rather than undermining them.
Labs that prioritize component quality are better positioned to maintain trust with their clinical partners.
Supporting Digital and Conventional Workflows
Modern laboratories often operate hybrid workflows that combine digital design with conventional techniques. Compatibility plays an important role here as well.
Accurate component geometry allows technicians to rely on digital libraries for CAD design while still achieving predictable fit during physical verification. This reduces the need for manual adjustments and shortens production timelines.
Compatibility also helps labs manage legacy cases where original digital files may not be available. In these situations, having access to reliable compatible components allows restorations to proceed without unnecessary delays.
Communication With Clinics
Managing compatibility is not just a technical issue. It also affects communication between labs and clinics. Clear identification of implant systems, connection types, and restorative goals is essential for predictable outcomes.
Labs that work regularly with compatible components often develop standardized intake processes that simplify case planning. This improves collaboration, reduces errors, and strengthens long-term relationships with referring clinics.
Adapting to a Changing Market
As implant dentistry continues to evolve, labs must remain flexible. New systems enter the market while older platforms remain in use for decades. Compatibility provides a way to bridge this gap, allowing labs to support a broad range of cases without constant operational changes.
For labs seeking to streamline restorative workflows across multiple implant systems, access to implant components compatible with widely used platforms, such as those different brands available at OEMDent Dental Implants, can help support efficient and predictable case management.
Final Thoughts
Implant compatibility has become an essential part of how dental laboratories operate in a multi-system environment. By reducing inventory complexity, supporting standardized workflows, and maintaining restorative accuracy, compatibility helps labs meet the demands of modern implant dentistry.
When managed correctly, compatibility allows laboratories to focus on what matters most, delivering reliable restorations that support long-term clinical success while operating efficiently in a competitive market.