The intersection of technology and healthcare is birthing revolutionary concepts, one of which is the use of blockchain technology in drug development.
This article delves into how the integration of blockchain into clinical trials can catapult advancements in drug development, providing a more robust, efficient, and patient-centric approach.
The focus is on the various aspects of this synergy: exploring its benefits, improving data accessibility and security, harnessing patient data for research, and ultimately empowering patients in the drug development process.
Key Points:
- Blockchain offers enhanced privacy, regulatory compliance, and patient control in clinical trials.
- Implementation of Blockchain can significantly improve data accessibility and security in drug development.
- Harnessing and analyzing patient data at a population scale can lead to the development of novel treatments and interventions.
- Various challenges, including limited accessibility and knowledge of clinical trial opportunities, need to be addressed to harness patient data fully.
- Blockchain technology has the potential to empower patients in the drug development process, leading to a more efficient care ecosystem.
Benefits of Blockchain in Clinical Trials
The benefits of utilizing blockchain in clinical trials include:
- Improved privacy
- Increased regulation enforcement
- Enhanced patient control over their own data
Blockchain used in healthcare, particularly in clinical trials, offers a decentralized and transparent platform for recording and sharing information. By leveraging cryptographic techniques, blockchain ensures the privacy and security of sensitive medical data.
Additionally, the use of smart contracts in blockchain allows for the enforcement of regulations and protocols throughout the clinical trial process. This reduces the risk of fraud and ensures the integrity of the data.
Furthermore, blockchain empowers patients by giving them control over their own data and allowing them to share it securely with healthcare providers and researchers.
Improving Data Accessibility and Security in Drug Development
Improving data accessibility and security is essential for facilitating efficient and secure sharing of medical information in the context of drug development. This subtopic explores various approaches and technologies that can address the challenges in data accessibility and security.
- Implementing blockchain technology can enhance data accessibility and security by providing transparency, trust, and disintermediation.
- Developing interoperable systems and standards can facilitate the aggregation and sharing of digital health records.
- Strengthening data privacy regulations and enforcing compliance measures can enhance the security of sensitive medical information.
- Educating patients about the benefits of sharing their medical information and providing incentives can encourage greater participation.
- Collaborating with industry actors and researchers to develop innovative solutions can improve data accessibility and security in drug development.
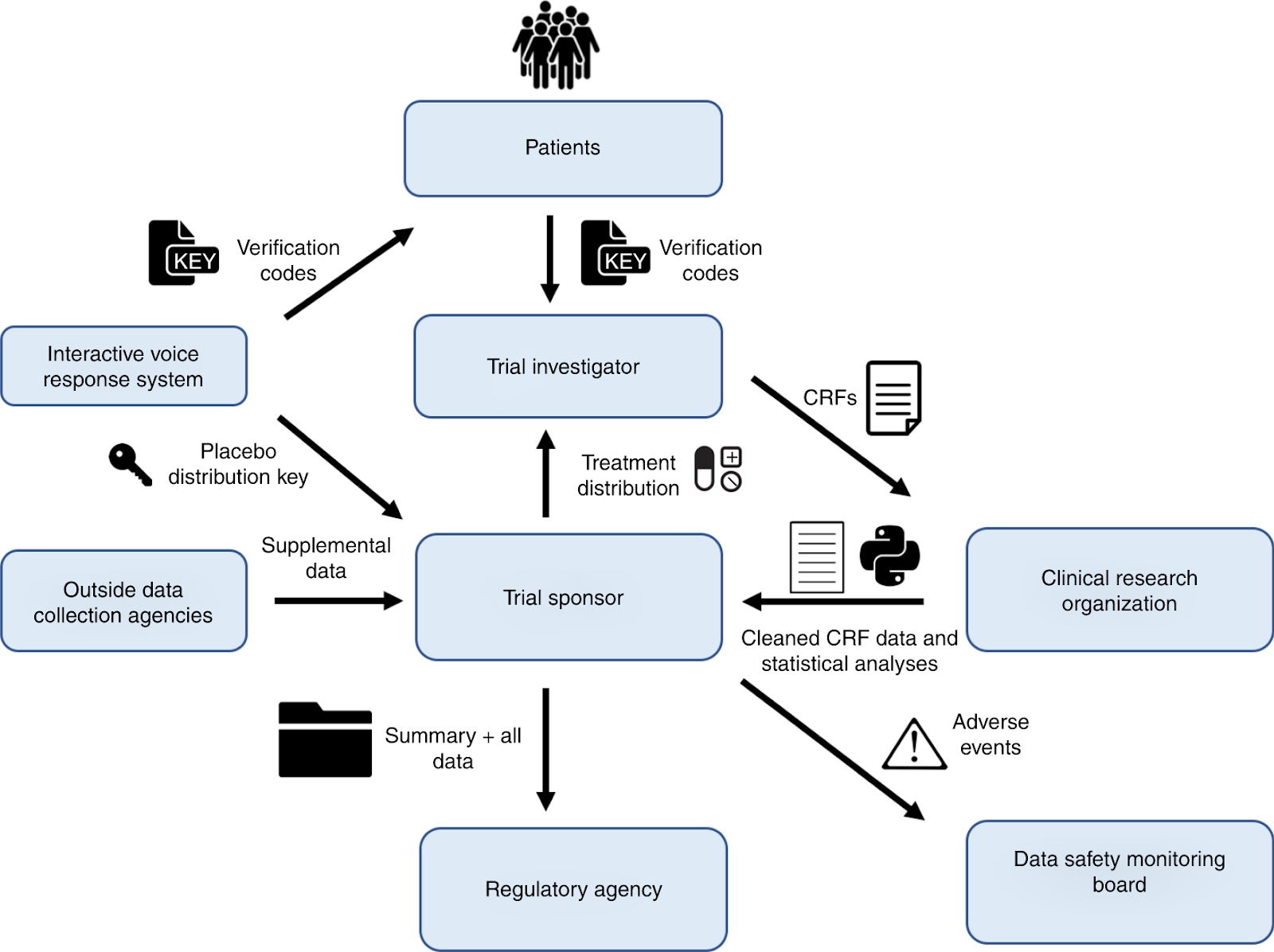
Harnessing Patient Data for Research and Development
Harnessing patient data for research and development involves leveraging the abundance of medical information collected by patients and utilizing it to advance scientific knowledge and improve healthcare outcomes.
Patient data, when aggregated and analyzed at a population scale, has the potential to provide valuable insights for research purposes.
By analyzing this abundant medical information, researchers can identify patterns, trends, and correlations that can contribute to the development of new treatments and interventions.
Additionally, patient data can also be used to evaluate the effectiveness and safety of existing therapies.

Empowering Patients in Drug Development Through Blockchain
Blockchain, with its capabilities of recording and sharing information in a transparent and secure manner, has the potential to empower patients in drug development.
By leveraging blockchain, patients can have greater control over their medical information and actively participate in the sharing and aggregation of data with physicians and researchers.
This can lead to a more efficient care ecosystem, where patient data is analyzed at a population scale for research purposes.
Through blockchain technology, the healthcare industry can be transformed into a patient-centric system, where patients are empowered in their health and well-being.
Conclusion
In summary, blockchain’s advent into clinical trials presents a transformative and promising opportunity to enhance the drug development landscape.
Through its potential to offer heightened data security, transparency, and patient control, it stands to redefine the traditional methodologies of drug development.
However, there are several challenges that need to be addressed, such as limited accessibility and knowledge of clinical trial opportunities, lack of incentives for patients to share their medical information, and concerns about the security and privacy of sensitive data.
Overcoming these barriers and harnessing patient data effectively can have a transformative impact on the healthcare industry.
As we continue to navigate the digital age’s promising potential, it becomes increasingly clear that blending technology with healthcare is a pivotal step towards a robust, efficient, and patient-centered future in drug development.
FAQs
Q: What is blockchain technology?
Blockchain is a digital, decentralized technology that records transactions across multiple computers to ensure the security and transparency of information. It utilizes cryptographic techniques to protect data from tampering, making it an ideal solution for the sensitive nature of clinical trials and drug development data.
Q: How does blockchain technology maintain data privacy in clinical trials?
Blockchain technology uses cryptographic techniques that encrypt and secure data. This ensures that personal and sensitive patient information used in clinical trials remains confidential and isn’t accessible to unauthorized individuals or entities.
Q: What are smart contracts and how are they used in blockchain?
Smart contracts are self-executing contracts with the terms of the agreement directly written into code. In the context of blockchain in clinical trials, smart contracts can automate and enforce the adherence to protocols and regulations, thereby reducing risks of fraud and ensuring data integrity.
Q: How does blockchain improve patient control over their data?
Blockchain allows patients to own and control their healthcare data. It provides a secure platform for them to share their data with healthcare providers and researchers, while maintaining privacy. With blockchain, patients can determine who can access their information, when and under what circumstances.
Q: What are some potential drawbacks of using blockchain in clinical trials?
Despite its many advantages, the use of blockchain in clinical trials could potentially face challenges related to scalability, interoperability with existing systems, and the need for sophisticated technology infrastructure. Furthermore, regulatory and legal issues concerning data handling and privacy, as well as the overall acceptance of the technology, also need to be considered.
Q: Are there any real-world examples of blockchain being used in clinical trials?
While the concept is still relatively new, there are ongoing efforts to incorporate blockchain into clinical trials. For instance, some pharmaceutical companies have initiated pilot programs to test its applicability and assess its potential benefits. Specific examples, however, would depend on proprietary information from those companies.
